-
Published: 16 January 2022

For emergency use for prevention of the Corona virus in Egypt, the Egyptian Ministry of Health has announced the approval of an Evochild license, especially for those whose bodies generate a limited immune response.
AstraZeneca Vaccines signed a contract with the Egyptian Standardized Procurement and Medical Logistics Authority of the Ministry of Health to supply the drug.
According to the Egyptian Medicines Authority statement, the first ephoshild doses are expected in Egypt in the coming weeks.
It is reported that EVOSHILD is used to protect vulnerable groups as a result of the inability of their bodies to generate an adequate immune response after receiving the Corona vaccine.
According to AstraZeneca, preliminary data created by clinical tests of the Omicron mutant demonstrated that Evochild retains effectiveness against all mutants of concern.
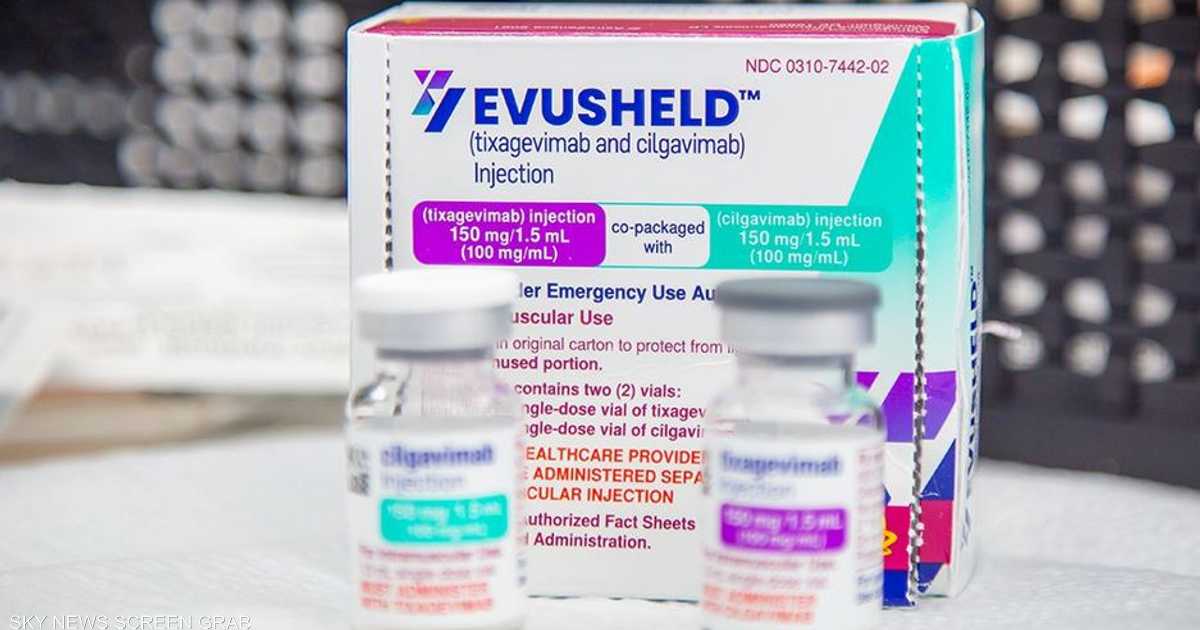
EVOSHIDE is a combination of Texa Gevimab and Silja Vimab from monogenomial antigen agents, which aim to raise immunity against Corona, and is jointly packaged and given as two syringes.
Amjad Al-Haddad, head of allergy and immunology at the Egyptian Serum and Vaccine Authority, comments that Evochild is a packaged antibodies ready for the new corona virus.
In special statements, he points out that the drug will be used for immunosuppressants, kidney patients, chemotherapists and vulnerable immunosuppressants.
The Head of the Sensitivity and Immune Section stresses that the drug will be used as a temporary alternative to the corona vaccine, granting long-term immunity for 6 months, and protecting against infection or complications in corona infection.
He explains that the drug resembles vaccines by giving a person anti-corona antibodies, as if he had received a vaccination dose.
On the possibility of taking the drug even after receiving the corona vaccine, he said that it was possible, as these ready antibodies contributed to an increased immune response within the body.
It should be noted that the Egyptian health spokesman, Hossam Abdel Ghaffar, had previously announced that three new drugs would arrive in Egypt last January.
He continued: "Three types of medication will arrive: Paxlovid produced by Pfizer, Molnoperavier produced by Merck and third by Evoschild.
According to Abdul Ghaffar, Egypt has terminated its contract with Pfizer to obtain doses sufficient to initially treat 20,000 people in the coming weeks.
He explained that Molnoperavir was an oral drug and had received emergency use approval from the American and European Drug Authority, noting that Egypt had contracted to supply doses of Evochild sufficient to treat 50,000 people.
On the other hand, the Egyptian Ministry of Health announced the receipt of 3 million doses of the corona virus vaccine produced by Pfizer, provided by the United States government.
Egypt has succeeded in providing all Corona vaccines in a short time, approved by the World Health Organization (WHO).
